ENGINEERING
PHYSICS – I L T P C
3 0 0 3
Aim: To explore various fundamental
aspects of Physics.
Objectives
·
To
understand the basic laws of physics and their applications in engineering and
technology.
·
To
develop scientific temper and analytical capability.
·
To
solve various engineering problems.
UNIT-I:
Acoustics 9
Introduction, sound waves - Pitch and Intensity. Reflection of sound
waves, sabine formula, absorption of
sound, reverberation Theory. Ultrasonics – production - magnetostriction oscillator and piezoelectric
oscillator. Properties and applications.
UNIT -II: Electron Optics 9
Introduction,
Electron-refraction-Bethe’s law, Electron Gun and Electron Lens. Cathode Ray
Tube and Cathode Ray Oscilloscope.
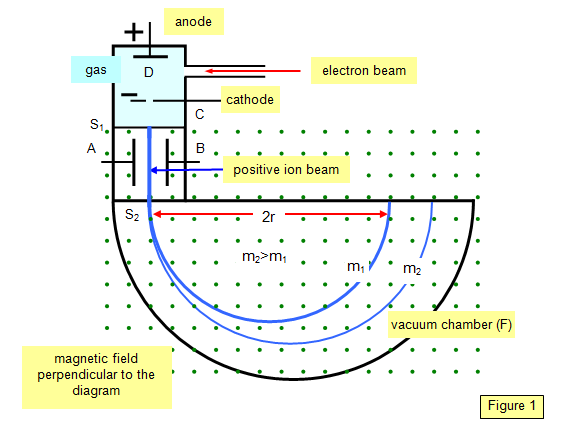
Cyclotron, Bainbridge Mass Spectrograph. Electron Microscope.
Applications.
UNIT
-III: Crystal structures and X-Rays 9
Introduction, Space lattice, unit cell, lattice parameters, Bravais
Lattice - Crystal systems. Characteristics of Unit cell (Cubic System). Miller
indices of planes. X-Rays –production, Bragg’s Law. Powder crystal method and
rotating crystal method.
UNIT
-IV: Band Theory of Solids 9
Introduction, Electrical conduction, conductivity, drift velocity,
influence of external factors on conductivity. The Band Theory of solids,
Energy Bands, Energy Gap. Classification of solids, Energy Band structure of a
conductor. Fermi-Dirac distribution function and Fermi Energy. Energy Band
structure of an Insulator and semiconductor.
UNIT
-V: Semiconductors 9
Introduction, Types- Intrinsic and
Extrinsic semiconductors. Intrinsic carriers-electron and hole concentrations.
Fermi level in intrinsic carrier density, Conductivity, Doping of
impurities-N-type and P-Type. Temperature variation-law of mass action-Charge
neutrality condition- Fermi level in extrinsic semiconductor-Hall effect.
Applications- Semiconductor diode, Transistor, FET, MOSFET.
Total:
45 periods
Beyond
the syllabus
Acoustics of buildings. Sonography,Cathode
Ray Tube,Magnetic bottle
Modes of laser beam,Classification of hologram,Bragg’s X-Ray
spectrometer,
Semiconductors in Electronics.
TEXT
BOOK
1.
M.N. Avadhanulu and P.G.
Kshirsagar ,A Text Book of Engineering Physics, S.CHAND and Co, 2012.
2.
Gaur and Gupta, Engineering
Physics , Dhanpat Rai publications, 2009
REFERENCES
1.
S.O.Pillai ,Solid State Physics,New age international
publications, 2010.
2.
M.Arumugam, Engineering Physics,Anuradha publications, 2009.
3.
Charles Kittel ,Introduction to Solid State Physics ,Wiley
India publications, 2009.
4.
Introduction to Solids
–L.Azaroff TMH,33rd Reprint
2009.
5.
Materials Science and
Engineering – William Calister – Wiley India- Sixth Edition 2009.